FertilityCare Calgary
Hidden
Charting with the Creighton Model FertilityCare System
Charting with the Creighton Model FertilityCare System
For achieving or avoiding pregnancy or evaluating gynecological health.
The Creighton Model FertilityCare™ System began its first Allied Health Education Program for FertilityCare™ Practitioners (FCP) in 1978. This means of objectively monitoring the biomarkers of the menstrual and fertility cycle was only in its beginning stages.
With the availability of the CrMS, observations of mucus patterns during the course of the menstrual and fertility cycle could be observed for the very first time. In fact, information obtained by women charting their cycles in this fashion is unique and can only be obtained in this fashion.
In Figure 40-2, a normal cycle with a normal mucus pattern, Peak Day and post-Peak phase is illustrated. In addition, the estrogen levels (black bars) and progesterone levels (red bars) are also shown to demonstrate the correlation to underlying hormonal events.

Figure 40-2, p. 511 from the NaPro textbook. A normal CrMS chart with daily levels of E2 and progesterone. The hormonal profiles are normal. The mucus cycle score is 15.7 and post-Peak phase is 10 days (From: Saint Paul VI Institute research, 2004).
Co-operative Treatment with Natural Procreative Technology
a) Real Solutions to Real Problems
This is the first women’s health science to network family planning with reproductive health monitoring and maintenance. It is a fertility-care based medical approach rather than a fertility-control approach to family planning and gynecological health.
- Infertility
- Menstrual cramps
- Premenstrual syndrome (PMS)
- Ovarian cysts
- Irregular or abnormal bleeding
- Polycystic ovarian disease
- Repetitive miscarriage
- Postpartum depression
- Prematurity prevention
- Hormonal abnormalities
- Chronic discharges
- Other health problems
Natural Procreative Technology (NaProTechnology) is a restorative reproductive medical model and surgical specialty for women of procreative age.
NaProTechnology (Natural Procreative Technology) is a women’s health science that monitors and maintains a woman’s reproductive and gynecological health. It provides medical and surgical treatments that cooperate completely with the reproductive system.
Thirty years of scientific research in the study of the normal and abnormal states of the menstrual and fertility cycles have unraveled their mysteries.
NaProTechnology uses the Creighton Model FertilityCare™ System biomarkers to monitor easily and objectively the occurrence of various hormonal events during the menstrual cycle. NaProTracking provides valid information that can be interpreted by a woman and by physicians who are specifically trained in this system.
Unlike common suppressive or destructive approaches, NaProTECHNOLOGY works cooperatively with the procreative and gynecologic systems. When these systems function abnormally, NaProTECHNOLOGY identifies the problems and cooperates with the menstrual and fertility cycles that correct the condition, maintain the human ecology, and sustain the procreative potential.
Women now have an opportunity to know and understand the causes of the symptoms from which they suffer.
b) Who developed Natural Procreative Technology?
Thomas W. Hilgers, MD, is the director of the Saint Paul VI Institute for the Study of Human Reproduction and the National Center for Women’s Health in Omaha, Nebraska. Working at the St. Louis University and Creighton University Schools of Medicine, Hilgers and his coworkers developed the Creighton Model FertilityCare System.
One of Dr. Hilgers’ pivotal discoveries is the standardized observation of menstrual and fertility cycles. Women chart with the Creighton Model FertilityCare System for gynecologic health maintenance, and couples use it to achieve or avoid pregnancy. Trained physicians can read a woman’s CrMS chart universally — this is the key to unraveling the mysteries of the menstrual and fertility cycle.
Dr. Hilgers is currently a senior medical consultant in obstetrics, gynecology, and reproductive medicine and surgery at the Saint Paul VI Institute. He is a clinical professor in the Department of Obstetrics and Gynecology at Creighton University School of Medicine, Omaha, Nebraska. In 1994, Dr. Hilgers was appointed to permanent membership to the Pontifical Academy for Life. In 2004, Dr. Hilgers published the definitive textbook on natural procreative technology, The Medical and Surgical Practice of NaProTECHNOLOGY.
c) Treatable Gynecolgical Conditions
1. Infertility
2. Endometriosis
3. Polycystic Ovarian Disease
4. Premenstrual Syndrome (PMS)
5. Post Partum Depression
6. Miscarriage and Preterm labour prevention pre-pregnancy and pregnancy monitoring
1. Infertility
Infertility
Treatable Gynecological Conditions
The graph below identifies conditions that are known causes of infertility and the success rates of treatment with NaPro.

Infertility is a symptom of underlying disease. The diseases that cause infertility have a “two-pronged” effect. They not only hinder the functioning of fertility, but they also cause both short and long-term health problems. The persistent unwillingness to address infertility problems from this point of view or perspective is one of the major flaws in the current approach to the treatment of infertility.
Fertility problems also carry with them significant emotional sequelae. This is fairly well recognized by those who work in this field and psychosocial distress can contribute significantly to the cause of some forms of infertility.
Until 1978, most of the effort in medicine in evaluating and treating women with infertility was placed in trying to identify and treat the underlying causes. In 1978, in vitro fertilization produced a paradigm shift. It led to a “skipping over” the causes and this continues up to the present time to be the foundational management approach. In essence, this is a symptomatic or Band-Aid approach to treatment, not one that gets to the root causes. When the artificial reproductive technologies began to take hold, now over 25 years ago, diagnostic laparoscopy was in its infancy. Hormone assessment, while available, was not readily accessible. Ultrasound technology was still mired in sector scanning and real-time ultrasonography was not yet available. Selective hysterosalpingography had not yet been developed and the fallopian tubes could not be catheterized.
Below is what a normal menstrual cycle and its correlating hormone profile would look like:

Figure 40-2, p. 511 from the NaPro textbook. A normal CrMS chart with daily levels of E2 and progesterone. The hormonal profiles are normal. The mucus cycle score is 15.7 and post-Peak phase is 10 days (From: Saint Paul VI Institute research, 2004).
Compare this to the next example, a completely dry cycle is observed. No mucus is observed during the course of the entire cycle. These dry cycle patterns occur in about 15 percent of all women with regular cycles and infertility. Looking at the underlying hormonal correlations (Figure 40-3), one can see that the estrogen and progesterone levels are significantly suboptimal compared to the levels observed in the normal cycle (Figure 40-2).
This suggests that the dry cycles – the absence of mucus – are a reflection of underlying hormonal abnormalities. We now know that this is an exhibition of abnormal folliculogenesis and abnormal luteogenesis (abnormal development of the follicle with a subsequent abnormal corpus luteum). This is also associated with abnormal ovulation events.

Figure 40-3 from p. 511 from the NaPro textbook. An infertility patient with a dry cycle and daily levels of E2 and progesterone. Both the preovulatory and the postovulatory profiles are very suboptimal revealing very poor folliculogenesis followed by abnormal luteogenesis (From: Saint Paul VI Institute research, 2004).
A NaProTechnology approach to the infertile couple has the following goals:
- It works towards assessing the underlying causes of the reproductive abnormality.
- It allows for the treatment of these underlying causes.
- It assists the couple in achieving pregnancy while maintaining the natural acts of procreation.
- If the treatment program is unsuccessful, research into the unknown causes is undertaken.
- If medically unsuccessful, the program will assist with successful family building by being supportive of adoption.
The use of a NaProTECHNOLOGY approach to infertility or miscarriage will also allow us to observe events that formerly had been ignored. An example of this might be that found in Figure 40-7. Here a woman with four consecutive miscarriages has charted her cycle. She has multiple days of premenstrual spotting prior to the beginning of her next period. The hormonal correlation shows a significantly reduced progesterone production by the corpus luteum suggesting an inadequate corpus luteum as one of the underlying causes of her repetitive miscarriages.

Figure 40-7 on p. 514 from the NaPro textbook. A woman with premenstrual spotting, a history of four consecutive spontaneous abortions and a preovulatory and postovulatory hormone profile. The periovulatory estradiol levels are decreased but what is most remarkable is the significant decrease in postovulatory progesterone (From: Saint Paul VI Institute research, 2004).
A staircase approach outlining the basic principles of NaProTECHNOLOGY for the evaluation and treatment of infertility is shown in Figure 40-8.

Figure 40-8 on p. 516 from the NaPro textbook. A staircase approach outlining the basic principles of a NaProTECHNOLOGY evaluation protocol for infertility. Evaluation and treatment protocol for infertility.
Several examples of successful outcomes in patients with infertility are shown in the next series of charts.
Case No. 1: In this patient with dry cycles and seven years of infertility, Vitamin B6 was recommended as a mucus enhancing supplement. In the last cycle, limited mucus was observed and pregnancy achieved without any additional assistance except fertility-focused intercourse (from Saint Paul VI Institute research, 2004).

Figure 40-24 on p. 537 from the NaPro textbook. In this patient with dry cycles and seven years of infertility, vitamin B6 was recommended as a mucus-enhancing supplement. In the last cycle, limited mucus was observed and pregnancy achieved without any additional assistance except fertility focused intercourse (From: Saint Paul VI Institute research, 2004).
Case No. 2: In this case, the patient, who previously lost sextuplets from an ART procedure at nearly 20 weeks of pregnancy, shows here CrMS chart which is strikingly consistent with polycystic ovarian disease (Figure 40-25).

Figure 40-25 from the NaPro textbook. This patient, who previously lost sextuplets from an ART procedure, shows her CrMS chart which is strikingly consistent with polycystic ovarian disease (From: Saint Paul VI Institute).
Case No. 3: The limited mucus cycles, endometriosis, ovarian dysfunction and a husband with a very low sperm count were identified in this patient who had failed two previous IVF procedures. In spite of these abnormalities, with proper NaProTECHNOLOGY treatment, she achieved a pregnancy and had a normal healthy baby (from Saint Paul VI Institute).

Figure 40-26 on p. 538 from the NaPro textbook. Limited mucus cycles, endometriosis, ovarian dysfunction and a husband with a very low sperm count were identified in this patient who failed two previous IVF procedures. In spite of these abnormalities with proper NaProTECHNOLOGY treatment, she achieved a pregnancy and had a normal healthy baby (From: Saint Paul VI Institute).
Using a NaProTECHNOLOGY approach for the treatment of infertility can be highly effective and even more effective than current approaches to infertility. In Figure 51-5, a life table comparison of the effectiveness of NaProTECHNOLOGY (in blue) with a similar non-NaProTECHNOLOGY approach taken at Johns Hopkins University is shown. The success rates are clearly better using the NaProTECHNOLOGY approach.

Figure 51-5, p. 682 from the NaPro textbook. Cumulative pregnancy rate of patients with endometriosis treated with NaProTECHNOLOGY compared to conservative surgery only. Patients with normospermic husbands only (From: Saint Paul VI Institute research, 2004 and Rock JA, Guzick DS, Sengos C, et al: The Conservative Surgical Treatment of Endometriosis: Evaluation of Pregnancy Success with Respect to the Extent of Disease as Categorized Using Contemporary Classification Systems. Fertil Steril 35:131-137, 1981).
A similar study showing a comparison of a NaProTECHNOLOGY approach to the treatment of women who have polycystic ovarian disease and comparing it to the work at Johns Hopkins University also shows a significant improvement (Figure 51-13).

Figure 51-13, p. 683 from the NaPro textbook. Cumulative pregnancy rate for patients with polycystic ovarian disease treated with NaProTECHNOLOGY compared to surgical wedge resection only. (From: Saint Paul VI Institute research, 2004 and Adashi EY, Rock JA, Guzick D, et al: Fertility Following Bilateral Ovarian Wedge Resection: A Critical Analysis of 90 Consecutive Cases of the Polycystic Ovary Syndrome. Fertil Steril 36:320-325, 1981).
In Figure 51-38, a comparison is made of the “per woman” pregnancy rates between the NaProTECHNOLOGY approach and in vitro fertilization. This study shows that a NaProTECHNOLOGY approach for women who have anovulatory infertility, polycystic ovarian disease, endometriosis, or tubal occlusion, all have statistically significantly higher pregnancy rates than patients with similar conditions treated with in vitro fertilization.

Figure 51-38, p. 691 from the NaPro textbook. The per woman pregnancy and family-building rates comparing NaProTECHNOLOGY and in vitro fertilization (From: Saint Paul VI Institute research, 2004 and the listed references).
2. Endometriosis
What is endometriosis?
- Debilitating gynecologic condition
- Presence of endometrial glands and stroma in ectopic locations
- 6-10% of reproductive aged women
- Associated with pain and infertility
- A disease marked by ectopic implantation of endometrial tissue in locations other than the intrauterine cavity
- 5 million American women at least
- Reactive tissue with inflammation and scarring
- Ovaries, tubes, bowels, outer surface of uterus and ovaries, other pelvic structures
- These tissues respond to hormones just as the endometrium normally responds
- Blood from the endometriosis lesions has no place to go
- Body tissues respond to this by:
- Surrounding it with inflammation
- This ends when menstrual bleeding ends
- Trying to absorb it back into the circulation
- Producing scar tissue
- Surrounding it with inflammation
- May cause adhesions, abnormal tissue that binds organs together
- Enough scar tissue can form tissue that is non responsive to hormones
- Patches that rupture spread contents to pelvic areas and allow it to spread
- Gradually worsens with time
- Good prognosis if surgery in young women
- May be hereditary
- Cancer rarely found with endometriosis
What are the risk factors for endometriosis?
- Severe pelvic pain, especially during menses—but not always
- Lower left quadrant common
- Many women have no symptoms at all
- Constipation/diarrhea at time of menses
- Dyspareunia
How is endometriosis diagnosed?
- Only by visual inspection of the abdomen and pelvis with histologic confirmation of biopsied lesions
- Near contact laparoscopy by reproductive surgical specialist
- A pelvic exam can help aid in diagnosis
- Ultrasound is generally not beneficial.

What are the signs of endometriosis?
- Ligh menstrual flow suggests abnormal progesterone
- Light period may be from endometriosis, hormones or receptors
- Poor mucus amount and quality
- Women with abnormal ovulations
- leads to light bleeding during period
- Premenstrual bleeding or Tail end brown (TEB) bleeding
- >3 days of L or VL flow may be a low grade infection
- TEB suggests low progesterone, low grade infection or thyroid
- Irregular sloughing of endometrium


If I have infertility, what are the chances of endometriosis?


Does endometriosis cause hormone imbalance?
- Hormonal dysfunction
- Suboptimal progesterone and estrogen levels post peak
- Endo and pelvic adhesions associated with low progesterone
- Suboptimal estrogen levels pre peak
- May have low LH levels pre peak and resistance to LH
- Suboptimal progesterone and estrogen levels post peak
What other underlying conditions go hand in hand with endometriosis?
- PMS: increased by endometriosis
- Infertility, miscarriage history
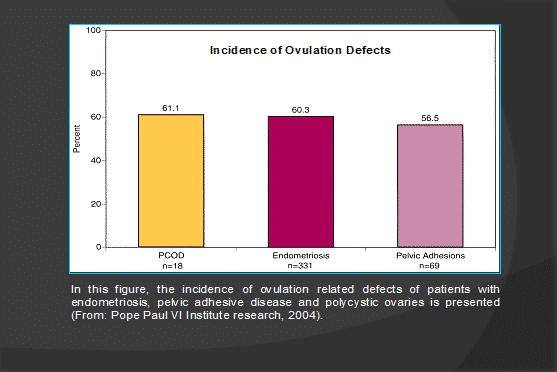
- PCOD: 50% of cases have endometriosis
- Pelvic adhesive disease
- Ovulation defects Luteal phase dysfunction: Type I, II, III
- Ovulatory dysfunction
- Miscarriage due to embryotoxins
- Diarrhea, constipation, IBS
- Autoimmune
- Inflammation

What treatment and help is available for endometriosis?
Restorative reproductive treatment with NaProTechnology includes various products including isomolecular hormones. Treatment of other endocrine related dysfunctions and laparoscopic surgery may also be part of the treatment plan.
3. Polycystic Ovarian Disease (PCOD)
Polycystic Ovarian Disease (PCOD)
According to American Family Physician, “Polycystic ovary syndrome is the most common endocrinopathy among reproductive-aged women in the United States, affecting approximately 7% of female patients. Although the pathophysiology of the syndrome is complex and there is no single defect from which it is known to result, it is hypothesized that insulin resistance is a key factor.
Metabolic syndrome is twice as common in patients with polycystic ovary syndrome compared with the general population, and patients with polycystic ovary syndrome are four times more likely than the general population to develop type 2 diabetes mellitus.
Patient presentation is variable, ranging from asymptomatic to having multiple gynecologic, dermatologic, or metabolic manifestations. Guidelines from the Endocrine Society recommend using the Rotterdam criteria for diagnosis, which mandate the presence of two of the following three findings—hyperandrogenism, ovulatory dysfunction, and polycystic ovaries—plus the exclusion of other diagnoses that could result in hyperandrogenism or ovulatory dysfunction. Because these findings may have multiple causes other than PCOS, a careful, targeted history and physical examination are required to ensure appropriate diagnosis and treatment.”
What are the signs of PCOD?
- Long, irregular cycles
- 95% of women with long cycles of 38 days will have PCOD, and many women who have intermediate long cycles over 32 days but less than 38 days
- Excessive amounts of cervical fluid during your cycle or cycles that are very dry
- Unusual spotting that occurs during the middle of the cycle
- Unusual hair growth in places where it shouldn’t be or hair loss
- Skin discoloration in the neck, armpit, under the breasts or around your navel or thighs
- Sleep apnea, snoring or insomnia
- Trouble with blood sugar


What does PCOD look like on a FertilityCare chart?

What other risk factors occur with PCOD?


Why is it important to treat PCOD?
Left untreated for long periods of time, PCOD is a risk factor for many serious conditions including breast and endometrial cancer due to the unopposed estrogen and suboptimal progesterone impact it has on the body.

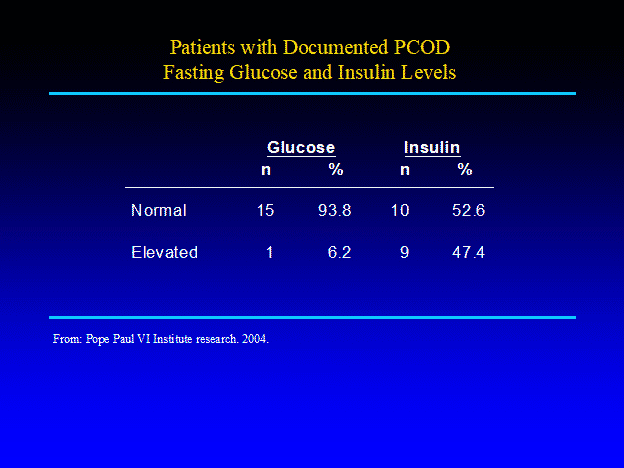
Can the ovulatory defects with PCOD be corrected?
Co-operative cyclical treatment with isomolecular progesterone can help correct the hormone levels to help establish a normal ovulatory event. This in turn will help create the proper hormone balance needed in the post peak phase of the cycle. Re-training the brain to reset the hormone pathways with progesterone is often all that is needed to improve symptoms and prevent long term complications.
Oral contraceptives or birth control pills may worsen the metabolic syndrome of PCOD and risks related to PCOD.
4. Premenstrual Syndrome (PMS)
Premenstrual Syndrome (PMS)
The condition now referred to as premenstrual syndrome (PMS) has a long and varied history among medical investigators. This history dates back to the time of Hippocrates, and the first reference in a scientific journal was by France in 1931. In 1964, Dr. Katherina Dalton brought attention to this condition with her first book on PMS, which promoted the theory that this condition was caused by either a progesterone deficiency or an imbalance in the estrogen-progesterone ratio. Later, she also extensively promoted the use of progesterone therapy for its treatment.
This condition has held back many women over the years. This should prompt interest and concern about finding the underlying causes and treating them effectively, so that those women who suffer from premenstrual syndrome are also given full access to opportunities. Furthermore, PMS is a condition that has destroyed relationships, led to divorce and child abuse and has created numerous aberrant stereotypes about the behavior of women.
The diagnosis of PMS includes the following list of symptoms:

The important aspect of diagnosis is that these symptoms must begin at least four days prior to the onset of menses. If they occur within three days of the onset of menses, they are considered to be normal premenstrual molimina. In addition to these 10 core symptoms, other symptoms have also been documented in this group of patients.
By charting one’s cycle, a physician can target the postovulatory phase of the cycle with an adequate hormonal evaluation. In women who have premenstrual syndrome, both progesterone and estrogen levels, along with beta-endorphin levels, are decreased late in the cycle. See Figures 29-11, 29-12, and 29-13.

Figure 29-11, p. 358 from the NaPro textbook.

Figure 29-12, p. 358 from the NaPro textbook.

Figure 29-13, p. 359 from the NaPro textbook.
By treating these hormonal abnormalities cooperatively with either cooperative progesterone replacement therapy or targeted HCG support (which should also improve both progesterone and estrogen production) and/or with the use of naltrexone as an opiate receptor antagonist, a high degree of success can be obtained with hormonal treatment.
At the present time, fluoxetine (Prozac) is considered the treatment of choice for women with premenstrual syndrome. However, in comparing targeted hormonal supplementation (cooperative progesterone replacement therapy) with Prozac, the targeted hormonal therapy is significantly more effective (Figure 29-16). In addition, the treatment effect is much more rapid in onset and helps attain the normal physiology of a woman so she actually feels more normal and does not feel “drugged.”

Figure 29-16, p. 362 from the NaPro textbook. The comparison of response to treatment of PMS with targeted HCG and progesterone hormone therapy versus 20 mg of fluoxetine hydrochloride versus placebo (From: Saint Paul VI Institute research, 2004, and published data on fluoxetine hydrochloride).
5. Post partum depression
Post Partum Depression
The earliest documentation of postpartum mental illness was provided by Hippocrates in 400 B.C. In spite of its evaluation over the years, postpartum depression (PPD) has remained an enigma. Pregnancy, miscarriage or pregnancy loss, infertility, and the postpartum period challenge a woman’s mental health. Virtually no life event rivals the hormonal, psychological and social changes associated with pregnancy and childbirth. Because of depressive episodes, up to 32 percent of women may alter their future childbearing plans by resorting to either adoption, sterilization or abortion.
At some point in their lives, 20 percent of women will suffer from depression. Many seek treatment from primary care providers, but up to 50 percent may go unrecognized and more go untreated. Recognition and treatment of depressive disorders in pregnancy and during the postpartum period is critical for the healthy outcomes of both the mother and infant.
Postpartum depression is identified as a major depressive disorder with postpartum onset. It is a major depressive episode that usually begins within the first four weeks following delivery. It can be extremely variable in both severity and duration.
Symptoms include the following: fatigue, changes in appetite or sleep, dysphoric mood, loss of interest in usually pleasurable activities, psychomotor agitation or retaliation, recurrent thoughts of death/suicide, feelings of worthlessness or guilt (especially failure at motherhood), and excessive anxiety over the child’s health.
Postpartum psychosis is a more severe postpartum syndrome. Its onset is usually within the first three weeks following delivery and often within just a few days. Most episodes are related to a psychotic condition of bipolar disorder or major depression. The symptoms include delusion, hallucinations, rapid mood swings ranging from depression and irritability to euphoria, sleep disturbances and obsessive ruminations about the baby. The risk of suicide in postpartum psychosis is high (up to five percent) and up to four percent of women with postpartum psychosis may attempt infanticide.
Postpartum psychosis is a psychiatric emergency that often warrants hospitalization. The prognostic implications are different from postpartum depression. Nearly two-thirds of these patients will suffer subsequent non-puerperal psychotic episodes.
Postpartum mood disorders are common, with nearly 40 percent (or more) of women experiencing them. The risk of psychiatric hospitalization within the first three months postpartum is seven times more common than at other times in a woman’s life.
https://naprotechnology.com/depression.htm – pagetop
Traditional Therapy
A traditional approach to therapy in this condition usually involves either psychotherapy or the use of antidepressant medications. In 1988, Dr. Katherina Dalton visited the Pope Paul VI Institute and during the course of that visit, she commented on her long experience with the use of progesterone in the treatment of postpartum depression. In addition, she seemed to think that postpartum depression was a very common problem.
These discussions prompted an interest in the use of progesterone support for the treatment of postpartum depression. Studies were then undertaken to understand the role of progesterone therapy for women with PPD.
The use of progesterone for the treatment of postpartum depression symptoms can be very dramatic. In Figure 30-3, the effects of progesterone on the treatment of depression, fatigue, crying, anxiety, helplessness, strange thoughts, poor appetite and night sweats were all statistically highly significant when progesterone was used. In fact, the incidence of these symptoms decreased significantly.

Figure 30-3, p. 377 from the NaPro textbook. Symptoms before and after progesterone therapy in patients with PPD (N=30) (From: Pope Paul VI Institute research, 2004).
In addition to the above, the average number of symptoms that a patient had relative to her postpartum depression were much higher before progesterone therapy than they were after. The number of symptoms experienced prior to progesterone therapy were 7.57 and this decreased, after progesterone therapy, to 2.1 (see Figure 30-5).

Figure 30-5, p. 378 from the NaPro textbook. The mean number of symptoms before and after progesterone therapy in patients with postpartum depression (N=30). (From: Saint Paul VI Institute research, 2004.)
There are multiple advantages to the use of progesterone. First of all, it is rapid in onset. Within literally minutes or hours after the first injection of progesterone, many of the symptoms are lifted. Secondly, over 95 percent of patients will respond positively to a progesterone therapy.
While progesterone therapy is not commonly used by obstetrician-gynecologists, this is mostly because of their lack of awareness of the effectiveness of progesterone in this situation. It is, bottom line, an incredibly effective treatment. It far exceeds the effectiveness of either psychotherapy or antidepressants and should be considered in the treatment of postpartum depression.
6. Miscarriage and Preterm labour prevention pre-pregnancy and pregnancy monitoring
Miscarriage and Preterm labour prevention pre-pregnancy and pregnancy monitoring (PCOD)
This program begins by identifying those patients who are at high risk for suboptimal progesterone, miscarriage, and going into preterm labor. These patients are taught how to self-monitor signs and symptoms of preterm labour.
Miscarriage or spontaneous abortion is defined as the spontaneous loss of pregnancy prior to the 20th gestational week of pregnancy. Pregnancy losses which occur during this period of time are said to occur in about 15 percent of pregnancies. At the same time, the risk of miscarriage increases proportionately to the number of previous miscarriages experienced.
Unfortunately, a definite cause has been difficult to determine. Over the years, miscarriages have been observed as a somewhat “normal” finding. Often it has been thought to be “nature’s way” of ending a pregnancy which was doomed to fail in any regard. However, there has developed a somewhat more aggressive approach over the last 5 to 10 years towards evaluation and management of women with spontaneous abortion. It is now well recognized that a definition of recurrent pregnancy loss includes two or more consecutive spontaneous miscarriages and that this warrants a full evaluation. Furthermore, it is becoming more and more recognized that there appears to be an association between infertility and spontaneous abortion.
A variety of factors underlie the occurrence of miscarriage. These include genetic, endocrinologic (hormonal), anatomic, immunologic and microbiologic variations. We are slowly coming to recognize that no miscarriage can be considered normal. All miscarriages are the result of a pathophysiologic reproductive event. It is the current challenge of medicine to find those underlying causes and, in some cases, underlying causes that are common occurrences are often overlooked.
In Figure 57-2, a Creighton Model chart in a woman who achieved a pregnancy and subsequently miscarried is shown. In this chart, various aspects of evaluation have been completed including hormonal and ultrasound studies. This woman clearly exhibits a “limited mucus cycle” as a biological marker of her Creighton Model cycle. Hormonally, she has a markedly decreased preovulatory estrogen profile and a markedly decreased postovulatory progesterone and estrogen profile. The follicle that was being monitored by ultrasound is also very small and is consistent with a condition known as the “Immature Follicle Syndrome.” Conception occurred in this cycle and ended in miscarriage. It has now been shown that those women who conceive and subsequently miscarry often have these limited mucus cycles.

Figure 57-2, p. 777 from the NaPro textbook. Conception in a limited mucus cycle leading to spontaneous abortion. An ultrasound study of the follicle revealed an immature follicle. Preovulatory E2 levels are suppressed and postovulatory P and E2 levels are suppressed. The mucus cycle is limited. The LH level is normal (from Saint Paul VI Institute research, 2004).
It has also been shown that women who have short post-Peak phases are also at risk for miscarriage. In Figure 57-3, a hormonal profile taken in a woman with a short post-Peak phase is shown. In this cycle, where the post-Peak phase is only five days in duration, the post-Peak or luteal phase is inadequate to support a pregnancy. Thus, if pregnancy occurs, the woman will miscarry. This condition is easily identified in a woman charting her cycles and, it can be easily treated, thus preventing miscarriage altogether.

Figure 57-3, p. 778 from the NaPro textbook. A CrMS chart showing a short post-Peak phase (five days) correlated with its hormone profile which shows a very short luteal phase (seven days). Pregnancy in this situation should universally lead to spontaneous abortion (From: Saint Paul VI Institute research, 2004).
In women with repetitive miscarriage, 85 percent will have endometriosis. This often goes unidentified because a diagnostic laparoscopy is not performed. However, such laparoscopies have been performed at the Saint Paul VI Institute in a large number of consecutive patients showing the presence of endometriosis and with subsequent treatment successful outcomes.
In addition, underlying hormonal abnormalities, ovulation defects and mucus cycle abnormalities can also be observed in patients with multiple miscarriages. The cumulative pregnancy rate of patients with a history of two or more spontaneous abortions in a NaProTECHNOLOGY-driven program is shown in Figure 57-22.

Figure 57-22, p. 793 from the NaPro textbook. A Kaplan-Meier life table curve comparing the cumulative pregnancy rates of patients with histories of either two or three spontaneous abortions. The pregnancy rates are not statistically significantly different (with treatment) (From: Saint Paul VI Institute research, 2004).

Figure 55-4, p. 731 from the NaPro textbook. The four zones of progesterone shown using the DPC-RIA assay.
Much of the work that has been accomplished on the assessment of serum progesterone in normal pregnancy was done from the early 1950’s through the 1970’s. Furthermore, most of the assessment of progesterone in pregnancy as it relates to various complications of pregnancy was accomplished from the early 1960s through the early 1980s. In spite of improvements in the accuracy and precision of progesterone assays since that time and a better ability to date pregnancy and establish more accurate gestational ages, very little subsequent work has been accomplished in this area.
However, data on the level of progesterone in normal pregnancy, and as it relates to a variety of pregnancy-related complications and features of previous reproductive history has been generated in a study which was conducted from the years 1980 through 2001 at the Pope Paul VI Institute. Modern means of progesterone assessment with improved accuracy and precision were used along with more precise means of dating the pregnancies.
In this study, 610 patients through 830 pregnancies and 8,545 progesterone levels were studied and statistically evaluated. These patients were primarily infertility patients who were receiving progesterone supplementation during the course of their pregnancy. Infertility was either primary or secondary and some of the patients also had a history of previous spontaneous abortion and, in some cases, recurrent spontaneous abortion.
The complete details of this study have been published in the textbook, The Medical & Surgical Practice of NaProTECHNOLOGY. A summary of the statistically significant changes in progesterone levels by condition or event is presented in Table 54-1.

Table 54-1, p. 720 from the NaPro textbook
It has been known for a long time that progesterone is decreased during the first trimester of pregnancy in patients who have spontaneous abortions. It has also been thought that the placenta takes over the production of progesterone during the second and third trimesters of pregnancy. This study shows that the level of progesterone in first and second trimester spontaneous abortions, are indeed statistically decreased. In fact, this observation supports the structure of the study conducted at the Pope Paul VI Institute.
However, a decrease in progesterone production, presumably by the placenta, during the second and third trimester of pregnancy in a variety of different pregnancy-related complications and previous pregnancy historical events was also observed. This suggests that the role of progesterone as an indication of placental function may be more significant than what had been previously appreciated and is especially important into the second and third trimesters of pregnancy. This stage of pregnancy has only been minimally studied in the past. While it is not new that a variety of pregnancy-related complications are associated with decreased progesterone production, in the study conducted at the Pope Paul VI Institute, there was an expanded evaluation of that very type of assessment. For example, it has been shown previously that serum progesterone levels have been noted to be decreased in intrauterine death, premature labor, threatened premature labor, premature rupture of the membranes, amnionitis and abruption of the placenta. Increased levels of progesterone have also been observed in twin pregnancies, Rh isoimmunization and hydatidiform mole. The last two conditions were not a component of the study at the Pope Paul VI Institute.
The data in the Institute’s very large and systematic assessment of progesterone in pregnancy suggests that the more significant progesterone-deficient time period in pregnancy is actually after the first trimester and into the second and third trimester. This is noted by comparing the sum of the pooled progesterone levels collected at six-week intervals during the course of pregnancies in a group of patients who had abnormal placentae, threatened prematurity and fetal distress (Figures 54-9, 54-10 and 54-11).

Figures 54-9, p. 721 from the NaPro textbook. Pooled six weekly data for progesterone in pregnancy: abnormal placental groups vs. normal pregnancy.

Figures 54-10, p. 721 from the NaPro textbook. Pooled six weekly data for progesterone in pregnancy: threatened prematurity group vs. normal pregnancy.

Figures 54-11, p. 721 from the NaPro textbook. Pooled six weekly data for progesterone in pregnancy: fetal distress group vs. normal pregnancy.
